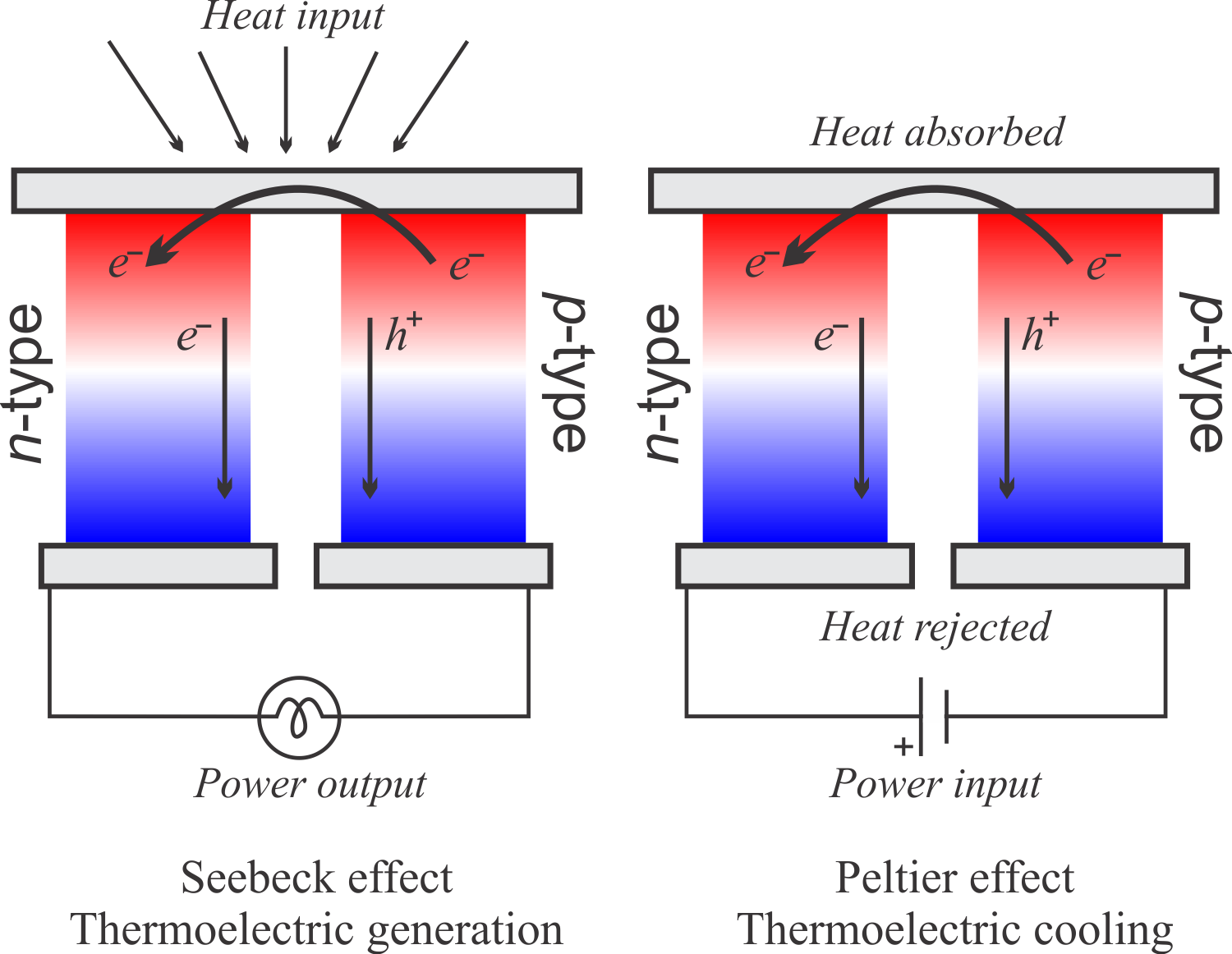
Thermoelectric Materials
Thermoelectric (TE) materials convert heat into electric energy and vice versa and as such may reduce
our dependence on fossil fuels. Thermoelectric materials can be used for a wide
range of applications such as Freon-free refrigerators and AC units, waste heat
and direct solar thermal energy converters. The performance of a TE material is
expressed by the dimensionless figure-of-merit ZT, ZT = TS2/kp,
where S is thermopower, T is the absolute temperature, p is electrical resistivity, and k is thermal conductivity. Thus, a TE
material should be a good electrical conductor (low p) and induce a high voltage in response to a temperature
gradient (high S), but should be a poor heat conductor to maintain the
applied temperature gradient (low k). State-of-the-art TE materials exhibit ZT values in the range of 1-2
depending on the application temperature. Further increases in ZT are
required for TE materials to become competitive with conventional heat exchange
systems. The main issue with engineering more efficient TEs arises from the
fundamental properties of solids: the parameters S, k, and p are strongly coupled and cannot be optimized
independently. Metals are inefficient TEs due to their high thermal
conductivity, while insulators exhibit very high electrical resistivity, which
decreases their ZT drastically.
The development of novel materials where charge and heat transport are partially de-coupled is a key factor
for the next generation of TEs. The "phonon glass-electron crystal (PGEC)" concept,
suggests the use of perfectly crystalline compounds containing loosely
bonded atoms inside oversized cages. These materials are expected to have low
thermal conductivity and low electrical resistivity. A widely studied class of
PGEC compounds are clathrates, a class of inclusion compounds containing a
three-dimensional framework with large cavities in which guest atoms are
situated. For the last 45 years about 200 tetrel clathrates were reported. The
term "tetrel" signifies that the frameworks of almost all clathrates are based
on Si, Ge, and Sn. Only a few exceptions were reported where a clathrate framework
was composed of late transition metals of groups 11-12 and pnicogens, group 15
elements. We believe that clathrates containing a high concentration of
transition metals in the framework will exhibit structural chemistry and
transport properties that are different from conventional tetrel-based
clathrates.
Our search for new thermoelectric materials has resulted in several compounds with unique crystal structures and
thermoelectric properties. We are continuing our work in this direction...