Zaikina Group
Research
Synthesis of oxides and semiconductors from sustainable solvents


Solvent choice plays a crucial role in the preparation of many technologically important materials. In synthesis of inorganic the sustainability is considered as one of the main components of the synthetic process. The particle size, crystallinity, morphology, atomic distribution and composition, micro and nanostructure play crucial role in the technological performance of functional materials. A judicious choice of synthetic parameters is particularly important for achieving desired characteristics. This project is devoted to the development of new low-temperature “green” synthetic routes to ternary oxide materials, as well as to binary chalcogenide and pnictide semiconductors.
Hydride route to complex intermetallics

Materials discovery can be accelerated by the development of synthesis methods and in-situ characterization techniques allowing for the rapid “fast screening” of multicomponent systems. However, the sluggish kinetics of solid-state reactions entails the necessity of high temperatures and long annealing times, often leading to the stabilization of the thermodynamically stable products. This becomes much more problematic when attempting to synthesize metastable and complex structures. Thus, new synthetic methods using unconventional, reactive precursors must be developed to overcome limitations of traditional solid-state synthesis. Such methods should be suitable to achieve the next level of synthetic complexity and allow exploration of systems where starting materials have drastically different reactivity. We are interested in unconventional synthesis methods toward solid-state materials, using reactive salt-like precursors. The chosen synthetic method utilizes alkali metal-hydride precursor and remedies the problem of insufficient mixing of the starting materials with ductile alkali metals, leading to faster kinetics and reduced annealing times. In addition, this route allows for targeted synthesis of specific compositions, and thus, fine tuning of physical properties. Such a synthetic approach could help bridge the gap between theoretical prediction and experimental synthesis of new materials, allowing for rapid exploration and compound mining of potentially rich phase space in the ternary systems containing an alkali metal.
Synthesis beyond thermodynamic limits: oxidative elimination route toward novel intermetallics
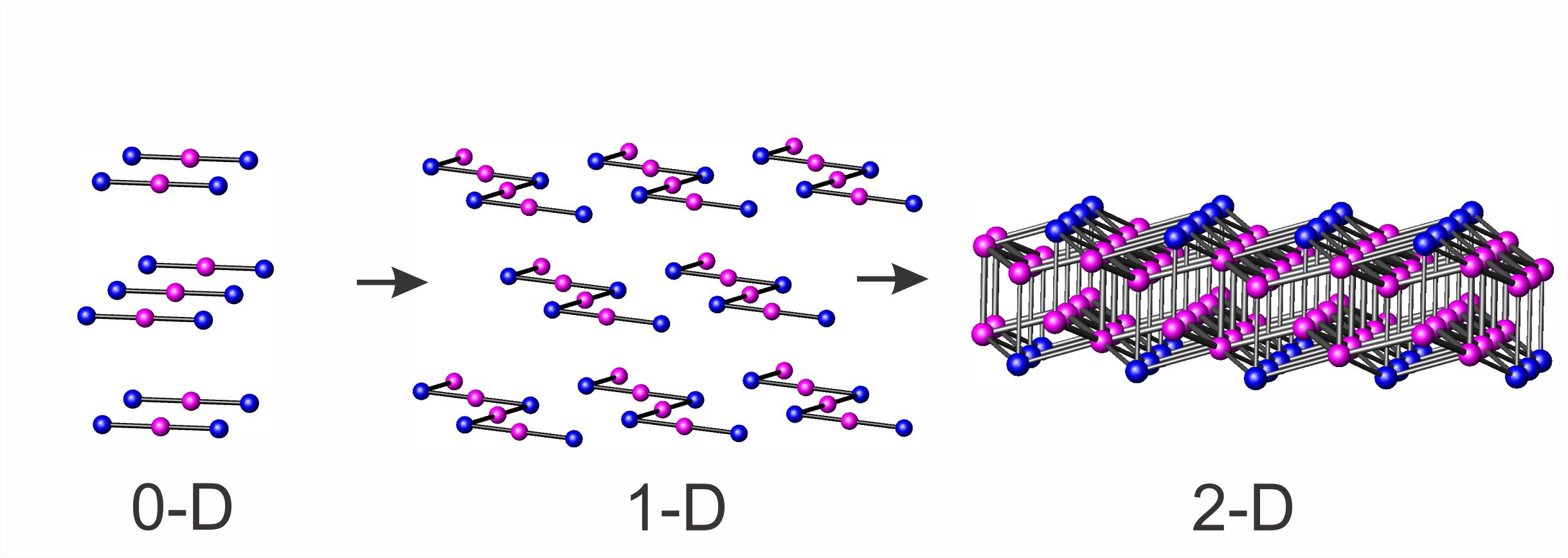
Compared to organic or traditional inorganic chemistry synthesis, where the molecular units can be linked to form the target product followed by the specific modification of the functional groups, the solid state chemistry synthetic routes are lacking the predictability of the synthesis outcome. The traditional high temperature solid state synthesis (“heat-and-beat”) is limited by slow diffusion in solids as well as restricted to the thermodynamically stable products. A large set of metastable or kinetically stabilized compounds, which are stable at elevated synthesis temperatures, are yet to be synthesized. Traditional synthetic methods are limited by low reactivity of elemental precursors at elevated temperatures. The ultimate goal of this project is to develop solid-to-solid transformation synthetic approach to access novel extended solids with unprecedented topology and properties.
 Contact:
Hach Hall, Office 2110, Lab 2221
Contact:
Hach Hall, Office 2110, Lab 2221