WINTER lab research
Research in our lab focuses on the development of new chemical tools for biological and medical applications. Philosophically, we take the view that many problems in biology are at heart problems in mechanistic physical organic chemistry. Using a joint theoeretical/experimental approach, we design and synthesize chemical tools for biological applications, including mediators for targeted drug delivery, biological sensors, and switch-on magnetic resonance probes. On the basic research side, we investigate the reactivity and properties of short-lived reactive intermediates in solution using high-level computational studies in combination with pulsed laser spectroscopy.
Photocages
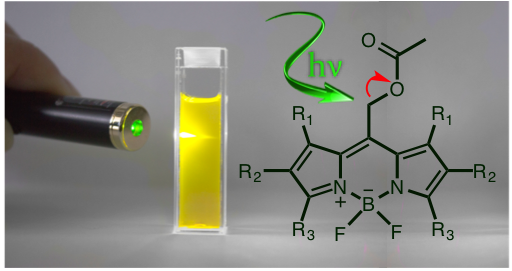
Photocages are light-sensitive chemical protecting groups that mask a substrate through a covalent linkage that renders the substrate inert. Upon irradiation, light is absorbed by the photocage and the substrate is released, restoring the substrate’s biological activity. These chemical tools are extremely valuable in biological settings because they allow investigators to control when, where, and how much of a bioactive substrate is released using targeted pulses of focused light. Released cargos can include drugs, dyes, ions, signaling agents, neurotransmitters, nucleotides, or bioactive small molecules.
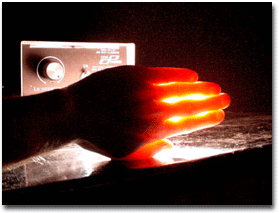
A drawback of most known photocages is that they absorb ultraviolet light. Irradiation with UV light is a problem for biological studies because UV light is phototoxic and does not penetrate far into tissue. The problem we are working on is developing chemical protecting groups that can release bioactive molecules using visible light, particularly with light wavelengths in the biological window where light penetration into tissue is maximal to allow photorelease in living systems (see graph above). Additionally, visible light is not phototoxic like UV light.
Red light penetrates furthest into tissue Representative Publications: Goswami, P., Beck, C., Zessin, T., Mahoney, K., Unash, R., Winter, A.H. “ BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light. ”2015, 139 (42), pp 15168–15175. |
Radical materials
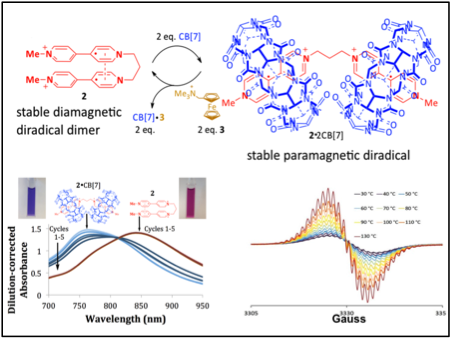
Interest in the synthesis of stimuli-responsive soft materials has led to a search for new strategies for achieving large property changes in organic materials in response to mild external cues. Our approach to this problem is to achieve stimuli-responsive changes in organic material properties by switching the spin state of stable organic radical-derived materials. Such spin crossover materials that change spin configuration upon a stimulus (e.g. heating) are established for inorganic molecules and transition metal complexes, but are essentially unprecedented for organic structures. Inorganic spin crossover materials find applications in thermochromic paints, sensors, displays, and mechanical actuators, and exploit the large changes in properties that occur upon switching spin states of a metal. Innovation Our key innovation has been to adapt the idea of spin crossover that has thus far been restricted to inorganic materials and bring this idea to organic materials. Specifically, we have identified organic free radicals based on viologen cation radicals that can switch between diamagnetic spin-paired forms and paramagnetic spin-unpaired forms upon different stimuli (e.g. temperature change, non-covalent binding, etc), leading to large changes in optical and magnetic properties. The key to our approach is to covalently link two stable organic radicals that form a weak pi bond (~2 kcal/mol) that leads to spin pairing of the two radicals. A stimulus that disrupts this very weak bonding interaction between the two radicals then leads to a spin-unpaired paramagnetic species, with large accompanying changes in optical and magnetic properties. These novel materials will lead to new classes of stimuli-responsive soft polymers and turn-on MRI contrast reagents that provide contrast upon activation by a biomolecule or other stimulus (pH, enzymatic activity, binding event, etc). Representative papers: Juetten, M., Buck, A., Winter, A.H. “A Radical Spin on Viologen Polymers: Organic Spin Crossover Materials in Water.” 2015, Chem. Commun. (Invited article for Emerging Investigators Issue) 51, 5516-5519 . |
oxenium ions
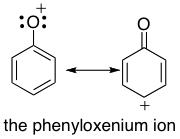
On the basic research side, we also investigate the reactivity and properties of oxenium ions, which are reactive intermediates that typically have sub-microsecond lifetimes in solution. These reactive intermediates are involved in numerous synthetically important reactions such as alkane and phenol oxidations and are intermediates in enzymatic oxidations of phenols. We investigate these reactive intermediates using a two-pronged theoretical-experimental approach, in which we compute properties of these ions that are difficult to obtain experimentally (such as electronic states), and then generate these reactive intermediates in solution using photochemical precursors to study their reactivity using ultrafast laser flash spectroscopy (in a collaboration with Prof David Lee Phillips, University of Hong Kong). Such photochemical precursors allow us to "see" these oxenium ions using laser spectroscopy, even though they only live in solution for the tiniest fraction of a second. These studies allow us to probe the reactivity of these intermediates in a quantitative way, and may allow for improved reactions involving these intermediates. Du, L., Qiu, Y., Lan, X., Zhu, R., Phillips, D., Li, M-D., Dutton, A., Winter, A.H. “Direct Detection of the Open-shell Singlet Phenyloxenium Ion: An Atom-Centered Diradical Reacts as an Electrophile.” 2017, J. Am. Chem. Soc. 139 (42), pp 15054–15059. Li, M, Hanway, P.J. , Albright, T.R., Winter, A.H, Phillips, D.L. "Direct Spectroscopic Observation of Open-shell Singlet, Closed-shell Singlet, and Triplet p-Biphenylyloxenium Ion". 2015, J. Am. Chem. Soc. 136 (35), pp 12364–12370 |

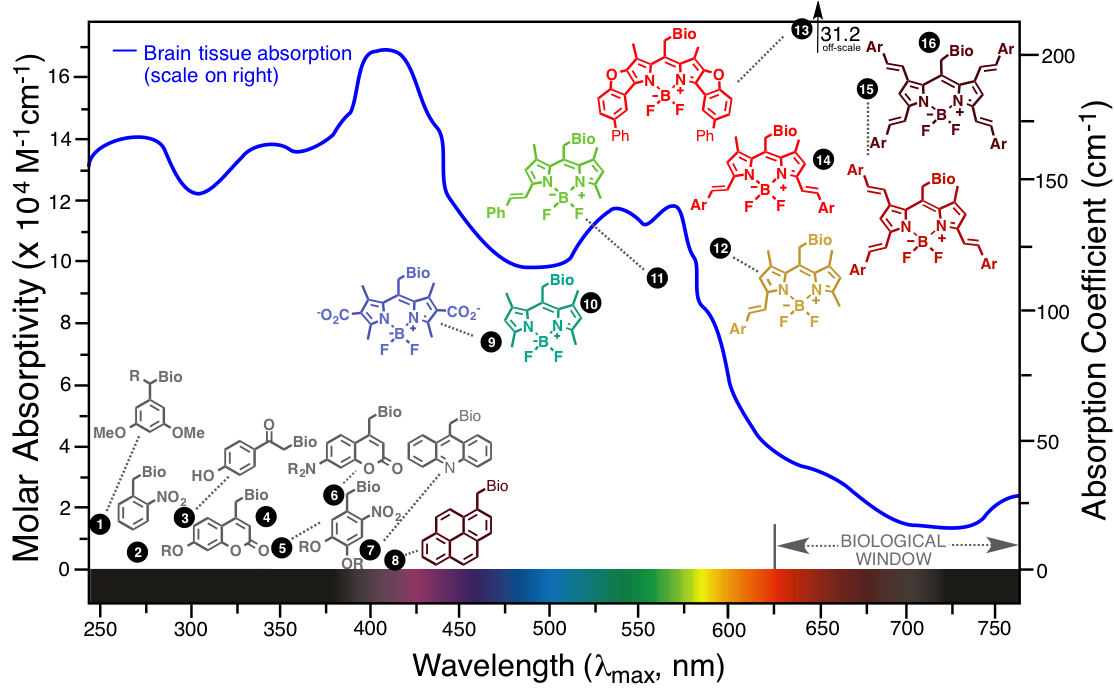 Plot comparing the optical properties of the most popular known photocages (1-8) with the new BODIPY photocages (9-16) being developed in our laboratory. The blue line shows the absorption of tissue (scale on right), demonstrating the location of the "optical window" where tissues are most transparent in the far-red/near-IR portion of the spectrum. These photocaging moieties release a bioactive molecule (Bio) upon absorbing light, allowing studies of biomolecules with spatial and temporal resolution in living systems.
Plot comparing the optical properties of the most popular known photocages (1-8) with the new BODIPY photocages (9-16) being developed in our laboratory. The blue line shows the absorption of tissue (scale on right), demonstrating the location of the "optical window" where tissues are most transparent in the far-red/near-IR portion of the spectrum. These photocaging moieties release a bioactive molecule (Bio) upon absorbing light, allowing studies of biomolecules with spatial and temporal resolution in living systems.