Thiel Research Group
Project Affiliations
Ames Lab - US DOEInnovative & Complex Metal-Rich Materials
Surface Structures Far-from-Equilibrium
IPRT - NSF
Formation and Stability of Supported Metal Nanostructures
Research Images
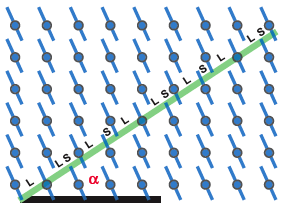
Quaicrystal periodicity
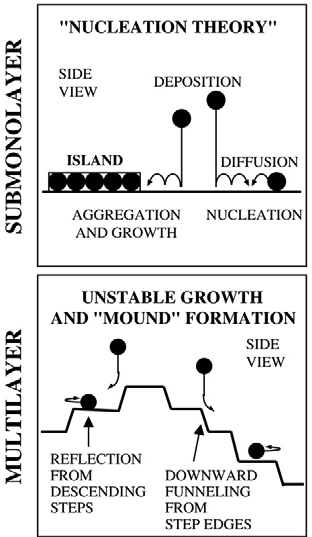
Thin film growth
Research Interests
1. Nucleation, growth, and intercalation of metal nanostructures at, and beneath, graphitic surfaces (funded by the Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering, through the Ames Laboratory); and
2. Adsorbate-metal complexes that form spontaneously on metal surfaces, and their effect on metal nanostructures (funded by the National Science Foundation, Division of Chemistry).
Metal Nanostructures on Semiconductors and Insulators
It is well known that properties of nanoparticles can deviate from properties of the bulk. Not only the size, but also the shape, is important. On surfaces, one striking example of this is the so-called Quantum Size Effect (QSE), which is an electronic stabilization of particular heights of metal particles. This is due to wavelength-matching between the wavelength of electrons semi-confined in the particle, and the height of the particle. The requirement of electron confinement means that this effect is seen most clearly if the metal particle is supported on a poorly-conducting surface. It is not clear whether special electronic properties of nanoparticles can affect chemical reactivity, especially in heterogeneous catalysis. We are studying model systems of metals on semiconductors, and metals on oxides, in order to determine this. An important auxilliary avenue of investigation concerns the kinetics and dynamics that influence particle shape and size in these systems. We are currently investigating silver (an important catalyst in ethylene epoxidation) on Si(111) surfaces. We recently found that the silver islands actually distort in order to maximize the match between the wavelength of electrons confined in the silver islands, and the dimension of the islands.
This work is supported by the U.S. Department of Energy, Office of Basic Energy Science, Division of Materials Sciences and Engineering.
Surfaces of Complex Metallic Alloys
Surfaces of complex metallic alloys / intermetallics, including quasicrystals, are important because these materials can display anomalous surface properties such as low friction, good oxidation resistance, and low surface energy. Often, however, basic information about these surfaces is lacking. We seek to answer the following questions. Do the surfaces retain the atomic and electronic structure of the bulk?and under what conditions? What are the most active adsorption sites? Can basic surface atomic and electronic structure be related to useful surface properties? How do these materials form interfaces with more conventional ones? Do they serve as templates for nanoparticles in unusual ways? How do they react in oxidizing environments? In the past, we have investigated quasicrystal surfaces extensively. (Quasicrystals are well-ordered, but non-periodic, at the atomic scale. Most of the known phases are Al-rich metallic alloys/intermetallics. They often exhibit a forbidden rotational symmetry, such as the five-fold symmetry element in an icosahedron.) As one example of our findings there, we established unabiguously that the low friction is dueat least in partto the unusual atomic structure. The expertise that has been established for studying quasicrystals can now be extended to studies of other complex systems, such as Gd-Ge, a prototypical material for magnetic refrigeration.
This work is supported by the U.S. Department of Energy, Office of Basic Energy Science, Division of Materials Sciences and Engineering.
The following article is a great introduction to quasicrystals:
P. A. Thiel, "Quasicrystal
Surfaces" Annual Review of Physical Chemisty, 59,
129-152 (2008).
Stability and Coarsening of Metal Nanostructures on Metal Surfaces
We are investigating the ways in which chemical additives destabilize metal nanostructures on metal surfaces. Specifically, the average particle size increases in a process called coarsening, a realization of the old axiom, "The big get bigger." In nanoscale science and technology, that's almost always a bad thing. We are exploring the effect of chemical additives in coarsening of metal islands on metal surfaces, and finding that they can accelerate coarsening rates by as much as three orders of magnitude. We seek a framework of understanding that encompasses different adsorbates, different metals, and different surface crystallographies, with particular emphasis on chalcogens (O, S, Se) as adsorbates and coinage metals (Cu, Ag, Au) as the metals. One effect that is particularly interesting is the formation of adsorbate-metal complexes that can be efficient carriers of mass between metal nanoparticles.
In a search for such complexes, we are teaming up with the research group of Yousoo Kim at RIKEN in Japan, and with theorists Jim Evans and Da-Jiang Liu at the Ames Laboratory. We focus on conditions of low adsorbate coverage, to isolate the complexes, and low temperature, to immobilize them. Under these conditions, we have discovered a number of exciting structures that form when sulfur adsorbs on coinage metals, including several complexes that simply defy the imagination. (In this context, a ‘complex’ is a stoichiometric mixture of metal and adsorbate atoms in which the metal is provided by the surface itself.) We are in the process of developing a broad understanding of when and why these complexes form.
This work is supported by the National Science Foundation.