Our long-term goal is to obtain a mechanistic, atomic-resolution understanding of the involvement of molecular dynamics in the regulation of catalytic systems. Major focus areas are enzymatic and heterogeneous catalyses, and are described below.
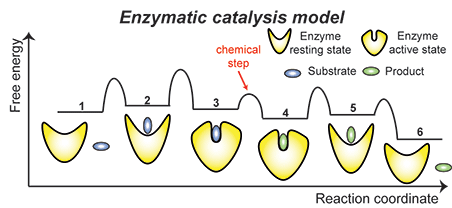 Enzymatic catalysis. Biological macromolecules sample a range of conformational states (or microstates). The distribution of this ensemble of microstates is dynamic and highly sensitive to changes in external conditions such as binding state, ligand concentration, pH, ionic strength and post-translational modifications. Important biological processes, including enzymatic catalysis, ligand binding, allostery, and signaling, depend on the exact composition of the ensemble and on interconversion rates between microstates. Therefore, understanding the delicate balance between structure and dynamics that governs biological function will increase our ability to manipulate or completely redirect protein function by selectively stabilizing particular conformations in the ensemble. Enzymatic catalysis. Biological macromolecules sample a range of conformational states (or microstates). The distribution of this ensemble of microstates is dynamic and highly sensitive to changes in external conditions such as binding state, ligand concentration, pH, ionic strength and post-translational modifications. Important biological processes, including enzymatic catalysis, ligand binding, allostery, and signaling, depend on the exact composition of the ensemble and on interconversion rates between microstates. Therefore, understanding the delicate balance between structure and dynamics that governs biological function will increase our ability to manipulate or completely redirect protein function by selectively stabilizing particular conformations in the ensemble.
We investigate the interplay between substrate/cofactor binding, conformational dynamics and biological function in enzymatic systems using a hybrid approach that combines theoretical data from Molecular Dynamics (MD) simulations, NMR experiments, and biochemical assays. In this approach, time-averaged and population-weighted NMR parameters (such as Paramagnetic Relaxation Enhancements, Residual Dipolar Coupling, order parameter, etc) are used to calculate conformational ensembles via restrained MD simulations, while kinetic information on the interconversion rates between microstates are obtained by NMR relaxation experiments. Finally, we employ a variety of biochemical methods to assay the effect of external perturbations of the structure, kinetics and thermodynamics of the conformational ensemble on the biological function of the enzyme under investigation.
We are currently interested in two different classes of enzymes: the histidine kinase Enzyme I (EI) of the bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS), and the human RNA demethylases FTO and Alkbh5.
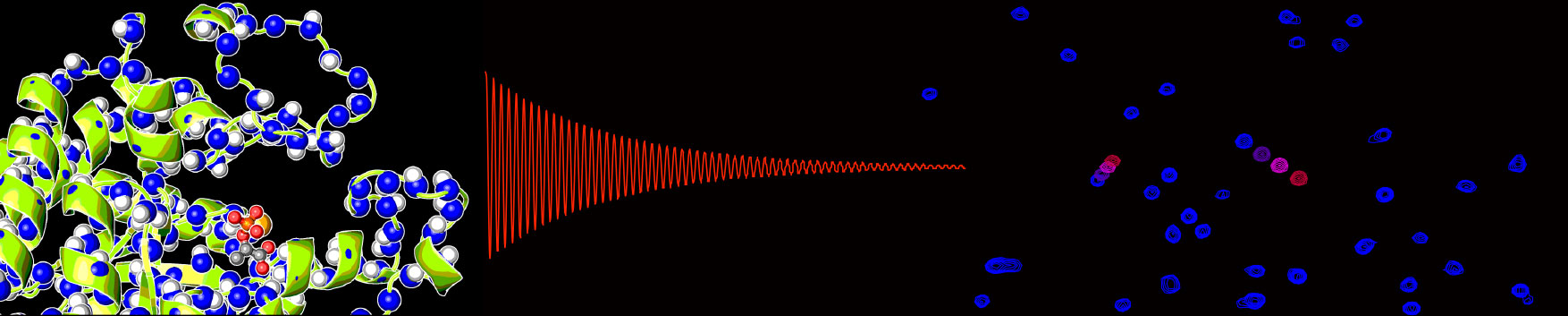
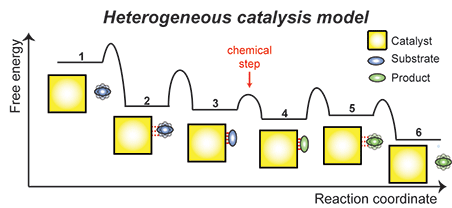 Heterogeneous catalysis. Heterogeneous catalysis can be modeled as a multistep process that proceeds through several intermediate states. The first step involves formation of an encounter complex between the catalyst and the substrate (2) that eventually evolves into a specific, catalytically competent interaction (3). At this stage, the chemical reaction takes place converting the substrate molecule into the product molecule (4), and the product is released from the catalyst surface (5, 6). If the catalytic process proceeds through intermediate compounds, two different scenarios can be imagined: 1) the catalytic site is recycled after the first catalytic step, or 2) the intermediate compound migrates to another catalytic site through the loosely bound state 5. In either model, the efficiency (i.e. the catalytic turnover and % conversion) and specificity of the catalyst depend on a complex free-energy landscape comprising several energy minima and transition states. We are using NMR and computational approaches to gain kinetic and thermodynamic information on each individual equilibrium on the free-energy pathway describing the catalytic process. In collaboration with the Slowing group at ISU, we are investigating how covalent and non-covalent surface modifications that modulate the molecular dynamics at the catalyst/solution interface affect catalytic performance and specificity. Heterogeneous catalysis. Heterogeneous catalysis can be modeled as a multistep process that proceeds through several intermediate states. The first step involves formation of an encounter complex between the catalyst and the substrate (2) that eventually evolves into a specific, catalytically competent interaction (3). At this stage, the chemical reaction takes place converting the substrate molecule into the product molecule (4), and the product is released from the catalyst surface (5, 6). If the catalytic process proceeds through intermediate compounds, two different scenarios can be imagined: 1) the catalytic site is recycled after the first catalytic step, or 2) the intermediate compound migrates to another catalytic site through the loosely bound state 5. In either model, the efficiency (i.e. the catalytic turnover and % conversion) and specificity of the catalyst depend on a complex free-energy landscape comprising several energy minima and transition states. We are using NMR and computational approaches to gain kinetic and thermodynamic information on each individual equilibrium on the free-energy pathway describing the catalytic process. In collaboration with the Slowing group at ISU, we are investigating how covalent and non-covalent surface modifications that modulate the molecular dynamics at the catalyst/solution interface affect catalytic performance and specificity.
|