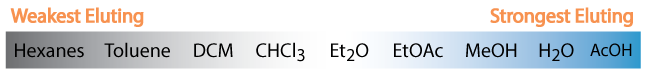How to TLC (Thin Layer Chromatography)
See Chapter 9.3 in Advanced Practical Organic Chemistry, 2nd ed. by Leonard, Lygo and Procter, 1998
- TLC is the simplest and quickest way to monitor a reaction and has the added benefit of helping to identify the best solvent system for flash chromatography.
- Each reagent should be spotted separatley and together (co-spot) along with the reaction mixture to aid identification. (co-spot).
- Monitor all reactions before adding the last reagent, during the reaction, and after the quench. If something goes wrong, you will not know when that happens unless you are monitoring the reaction.
- A good solvent composition is one that moves all components of your mixture off the baseline, but does not put anything on the solvent front - Rf values between 0.15 and 0.85 (this may not always be possible). See Flash Chromatography section for more on solvents.
- If two spots are extremely close, then spot them twice (see picture). If a Z-pattern develops (below), they are NOT the same material.
- Visualization: (1) By sight (2) UV (3) Stains (see below).
- For streaky amines, add 0.5% Et3N to the solvent chamber. For streaky acids, add 0.5% AcOH.
- Draw the TLC plate in your notebook and mark with observations.
- Reaction mixtures likely need to be diluted before spotting. If your spot streaks it is too concentrated.
- For more detailed instructions see the reference above and here
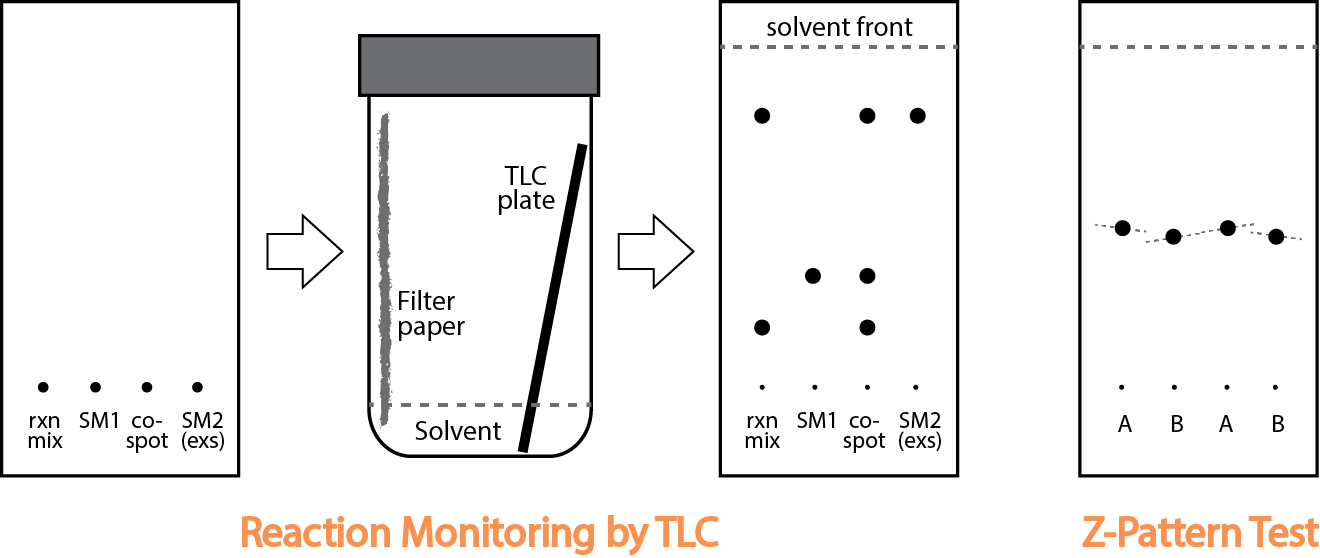
How to Perform Flash Chromatography
See Chapter 11.6 in Advanced Practical Organic Chemistry, 2nd ed. by Leonard, Lygo and Procter, 1998
- Pick a solvent pairing (often informed by TLC reaction monitoring). The most popular pairing is EtOAc:Hexanes, but any combination from the elution series below can work. Other popular pairings are DCM:Hexanes, DCM:EtOAc, DCM:MeOH (Note: eluting power does not always correlate with polarity)
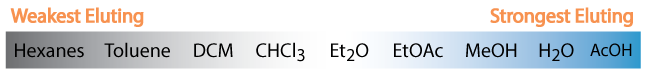
- Select your solvent composition such that your desired spot has an Rf=0.1~0.3 by TLC depending on separation (lower for close spot separations, higher for larger separations).
- Slurry the silica in your eluting solvent and pack the column under positive pressure and add a layer of sand on top so the silica gel is not disturbed by subsequent additions.
- Dissolve your reaction mixture in a minimum ammount of eluting solvent and gently add to the top of the column.
- Many times your mixture is not soluble in your eluting solvent. Instead dissolve the sample in a minimum of any good solvent of your choosing (DCM, THF, etc...). Add dry celite to the solution to form a coated powder. Rotovap to absorb onto the celite and give a free-flowing, dry powder (might need to scrape free with a spatula). Add this solid to the prepacked silica gel column and then add sand. Run as usual.
- Apply pressure to ensure a fast flow rate.
- Change to more polar solvent composition over time if necessary for good separation.
- For streaky amines, add 0.5% Et3N to the eluting solvent. For streaky acids, add 0.5% AcOH.
- Collect fractions into test tubes and monitor the progress of the separation in real-time using TLC. How many fractions can you fit on one TLC plate? Run the TLC plate halway and flip it over to use the other half to double your numbers!
- For small scale reactions, a glass pipette can serve as a column, but preparative TLC is often more reliable.
Recipes for TLC Stains
(Adapted from Prof. Justin DuBois, Stanford University)
More stains and preps here and here
Potassium Permanganate
oxidizable groups: alcohols, aldehydes, amines, double bonds, but heat the plate and you can stain almost anything
| 300 mL |
Deionized water (add in order) |
| 2 g |
KMnO4 (fully dissolve) |
| 20 g |
K2CO3 |
| 5 mL |
5% NaOH |
10-Hydroxybenzo[h]quinoline (HBQ)
Highly selective for boronic acids; image with 365 nm light. Highly electrophilic groups (eg. RCOCl) may also stain. Org. Lett. 2013, 15, 5382.
| 100 mL |
Absolute ethanol |
| 20 mg |
10-Hydroxybenzo[h]quinoline (HBQ) |
CAM (Ceric Ammonium Molybdate)
hydroxyl groups, carbonyl groups, peptides/amides
| 4 g |
Cerium sulfate (complex with H2SO4) |
| 100 g |
Ammonium molybdate tetrahydrate |
| 900 mL |
Deionized H2O |
| 100 mL |
Conc. H2SO4 |
Iodine
best for unsaturated compounds, spots may fade over time
| 500 mg |
I2 |
| 20 g |
silica |
| |
Amber jar |
Ninhydrin
sensitive and selective detection of primary amines, heat to stain BOC-protected amines, weak for secondary amines
| 3 g |
Ninhydrin |
| 30 mL |
Glacial acetic acid |
| 970 mL |
n-butanol |
| |
Stir until dissolved and store in brown bottle in the freezer |
PMA (Phosphomolybdic Acid)
general stain
| 10 g |
PMA |
| 127 mL |
95% EtOH |
| |
Stir until dissolved and store in freezer |
Vanillin
general stain, range of colors, smells great
| 250 mL |
EtOH |
| 15 g |
Vanillin |
| 2.5 mL |
Conc. H2SO4 (add dropwise) |
Anisaldehyde
general stain, range of colors
| 2.5 mL |
AcOH |
| 6.5 mL |
p-Anisaldehyde |
| 300 mL |
Ice cold 95% EtOH (or EtOAc, not denatured EtOH) |
| 8.5 mL |
Cautiously add conc. H2SO4 dropwise over 60 min. |
| |
Store unused portions in the freezer |
SPPS Lab Procedures
Adapted from Fmoc Solid Phase Peptide Synthesis: A Practical Approach, Edited by W. C. Chan and P. D. White, 2000
Manual Peptide Synthesis
- Complete this background knowledge problem set that describes the chemistry behind SPPS.
- Read the first three chapters of the above Chan and White reference.
- Download this excel file to assist in method preparation.
- Synthesize peptide!
Cleavage
- Transfer the dry resin into a peptide cleavage funnel. Use DCM to aid in resin transfer and to rinse all traces of DMF by filtration. (small scale test cleavage funnels)
- Add cleavage cocktail (see below) and leave to stand at room temperature with occassional swirling for 1.5-18h, depending on sequence.
- Filter the resin and collect the filtrate in a 50 mL conical tube (tube A). Wash the resin twice with fresh TFA. Keep the expended resin in case the cleavage was not complete.
Cleavage Cocktails
| |
Cocktail |
Time |
Peptides containing all amino acids except Arg(Mtr/Pbf/Pmc), Cys(Trt), Met, and unprotected Trp
|
TFA/TIPSH/water
95:2.5:2.5 (v/v)
|
1.5-3 h
|
Peptides containing all amino acids except Arg(Mtr/Pbf/Pmc), and/or unprotected Trp
|
TFA/TIPSH/water/EDT
94:1:2.5:2.5 (v/v)
|
1.5-3 h
|
All peptides
(time depends on number of Arg) |
TFA/thioanisole/water/phenol/EDT
82.5:5:5:5:2.5 (v/v) |
1.5-18 h |
Isolation
- Fill two 50 mL conical tubes (tube B and C) with ether and place them in the dry ice bin.
- Evaporate the TFA filtrate (tube A from step 3) by blowing N2 over the surface of the liquid via a needle. Immerse the end of tube A in a beaker of warm water to aid evaporation. Reduce the volume to obtain a glassy film.
- Add 50 mL of cold ether (tube B from step 4) to the evaporated residue and centrifuge (use tube C from step 4 as the counterbalance).
- Decant the ether, taking care not to disturb the pellet of precipitated peptide, and repeat the washing and centrifugation by adding fresh cold ether (tube C from step 4). Use the decanted waste ether as the new counterbalance. Save the waste ether portions and store in the freezer overnight. More peptide may precipitate.
- Allow the peptide to air-dry and confirm desired peptide is present by MALDI. Store in freezer until purification by HPLC.