-
HOME
-
RESEARCH
-
GROUP
-
PUBLICATIONS
-
PHOTOS
-
OPPORTUNITIES
-
CONTACTS
-
LINKS
Published at Iowa State University
59. Volkov, A. Mi, J.; Lalit, K.; Chatterjee, P.; Jing, D.; Carnahan, S.; Chen, Y.; Sun, S.; Rossini, A. J.; Huang, W.; Stanley, L. M. “General Strategy for Incorporation of Functional Group Handles into Covalent Organic Frameworks via the Ugi Reaction” J. Am. Chem. Soc. 2023, 145, 6230-6239. DOI: 10.1021/jacs.2c12440
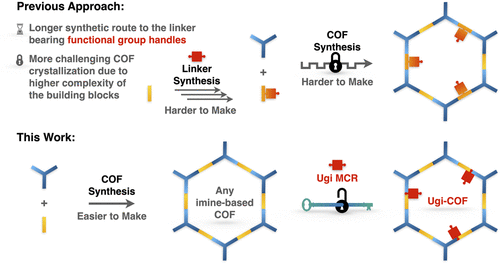
59. Volkov, A. Mi, J.; Lalit, K.; Chatterjee, P.; Jing, D.; Carnahan, S.; Chen, Y.; Sun, S.; Rossini, A. J.; Huang, W.; Stanley, L. M. “General Strategy for Incorporation of Functional Group Handles into Covalent Organic Frameworks via the Ugi Reaction” J. Am. Chem. Soc. 2023, 145, 6230-6239. DOI: 10.1021/jacs.2c12440

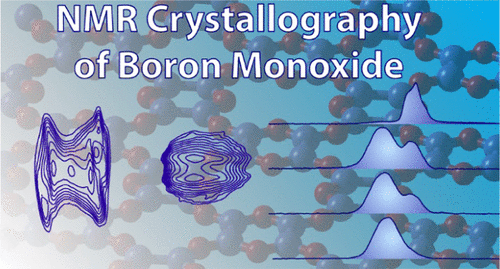
58. Perras, F. S.; Thomas, H.; Heintz, P.; Behera, R.; Yu, J.; Viswanathan, G.; Jing, D.; Southern, S. A.; Kovnir, K.; Stanley, L.; Huang, W. “The Structure of Boron Monoxide” J. Am. Chem. Soc. 2023, 145, 14660-14669. DOI: 10.1021/jacs.3c02070
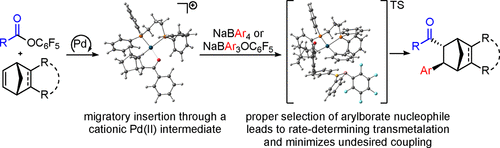
57. Alkan, M.; Banovetz, H. K.; Gordon, M. S.; Stanley, L. M. “Computational and Mechanistic Studies of Pd-Catalyzed Alkene Carboacylation via Ester C-O Bond Activation: ACS Catal. 2023, 13, 9766-9776. DOI: 10.1021/acscatal.3c01405
56. Youmans, D. D.; Tran, H. N.; Stanley, L. M. “Nickel-Catalyzed Isomerization of Homoallylic Alcohols” Org. Lett. 2023, 25, 3559-3563. DOI: 10.1021/acs.orglett.3c01201
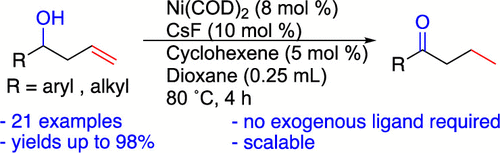
55. Moore, A. S.; Stanley, L. M. “Nickel-Catalyzed Formation of α-Substituted γ-Amino Ketones via Alkene Carboacylation” Org. Lett. 2022, 24, 8959-8963. DOI: 10.1021/acs.orglett.2c03413
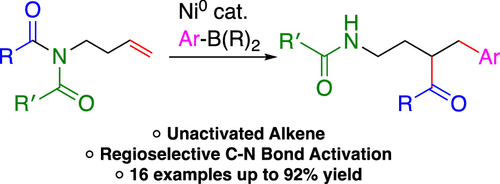
54. Tran, H. N.; Nguyen, C. M.; Koeritz, M. T.; Youmans, D. D.; Stanley, L. M. “Nickel-Catalyzed Arylative Substitution of Homoallylic Alcohols” Chem. Sci. 2022, 13, 11607-11613. DOI: 10.1039/d2sc01716d
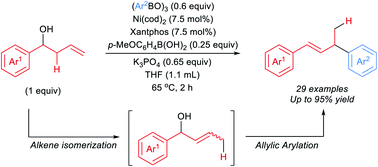
53. Koeritz, M. T.; Banovetz, H. K.; Prell, S. A.; Stanley, L. M. “Synthesis of Oxaboranes via Nickel-Catalyzed Dearylative Cyclocondensation” Chem. Sci. 2022, 13, 7790-7795. DOI: 10.1039/d2sc01840c
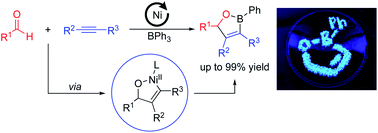
52. Tran, H. N.; Stanley, L. M. “Nickel-Catalyzed Enantioselective Hydroboration of Vinylarenes” Org. Lett. 2022, 24, 395-399. DOI: 10.1021/acs.orglett.1c04073
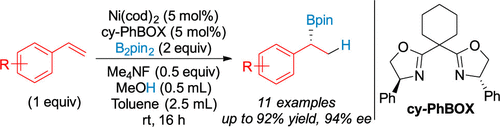
51. Kadam, A. A.; Metz, T. L.; David, C. M.; Koeritz, M. T.; Stanley, L. M. “Palladium-Catalyzed Intermolecular Acylative Heck Reactions with Imides as Acyl Electrophiles” J. Org. Chem. 2021, 86, 6863-6868. DOI: 10.1021/acs.joc.1c00087
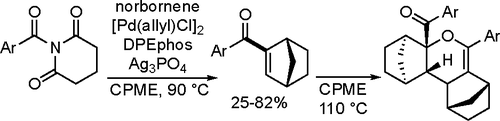
50. Banovetz, H. K.; Vickerman, K. L.; David, C. M.; Alkan, M.; Stanley, L. M. “Palladium-Catalyzed Intermolecular Alkene Carboacylation via Ester C-O Bond Activation” Org. Lett. 2021, 23, 3507-3512. DOI: 10.1021/acs.orglett.1c00940
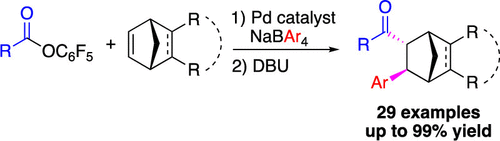
49. Tran, H. N.; Burgett, R. W.; Stanley, L. M. “Nickel-Catalyzed Asymmetric Hydroarylation of Vinylarenes: Direct Enantioselective Synthesis of Chiral 1,1-Diarylethanes” J. Org. Chem. 2021, 86, 3836–3849. DOI: 10.1021/acs.joc.0c02556
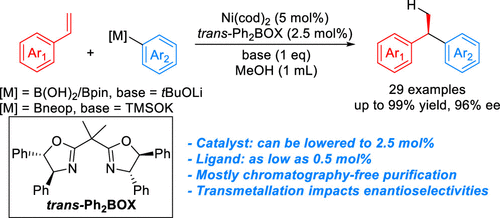
48. Koeritz, M. T.; Burgett, R. W.; Kadam, A. A.; Stanley, L. M. “Ni-Catalyzed Intermolecular Carboacylation of Internal Alkynes via Amide C–N Bond Activation” Org. Lett. 2020, 22, 5731–5736. DOI: 10.1021/acs.orglett.0c01607
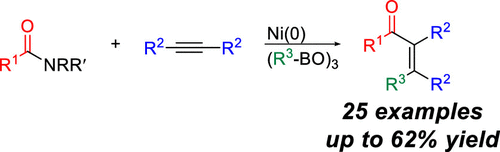
47. Heintz, P. M.; Schumacher, B. P.; Chen, M.; Huang, W.; Stanley, L. M. “A Pd(II)-Functionalized Covalent Organic Framework for Catalytic Conjugate Addition of Arylboronic Acids to β,β-Disubstituted Enones” ChemCatChem. 2019, 11, 4286-4290. DOI: 10.1002/cctc.201900894
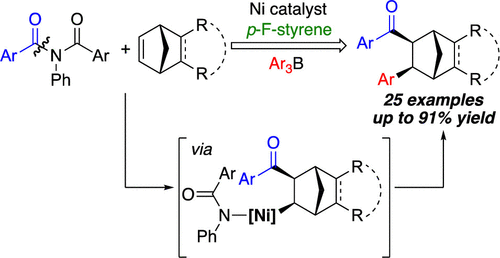
46. Kadam, A. A.; Metz, L. T.; Qian, Y.; Stanley, L. M. “Ni-Catalyzed Three-Component Alkene Carboacylation Initiated by Amide C-N Bond Activation” ACS Catal. 2019, 9, 5651–5656. DOI: 10.1021/acscatal.9b01620
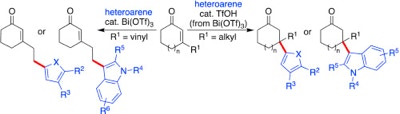
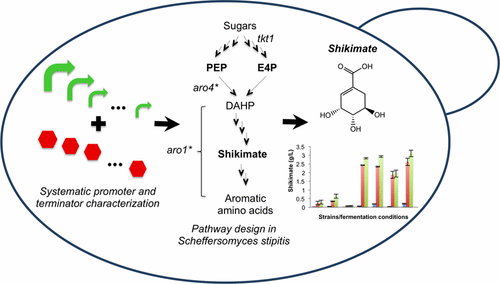
45. Metz, L. T.; Leng, M.; Evans, J.; Stanley, L. M. “Synthesis of heteroarylated ketones via bismuth(III) triflate-promoted regioselective 1,4- and 1,6-additions of electron-rich heteroarenes to cyclic enones and dienones” Tetrahedron. 2018, 26, 328-3292. DOI: 10.1016/j.tet.2018.04.002
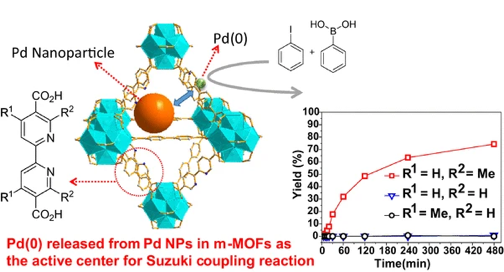
44. Li, X.; Zhang, B.; Van Zeeland, R.; Tang, L.; Pei, Y.; Qi, Z.; Goh, T. W.; Stanley, L. M.; Huang, W. “Unveiling the Effects of Linker Substitution in Suzuki Coupling with Palladium Nanoparticles in Metal Organic Frameworks” Catal. Lett. 2018, 148, 940-945. DOI: 10.1007/s10562-017-2289-9
43. Metz, L. T.; Lutovsky, A. G.; Stanley, L. M. “An Acid-Catalyzed Addition and Dehydration Sequence for the Synthesis of Heteroarylated Steroidal Dienes” J. Org. Chem. 2018, 83, 1643–1648. DOI: 10.1021/acs.joc.7b03045
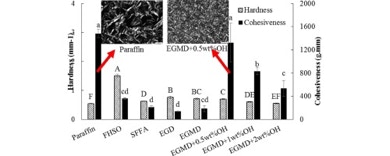
42. Fei, T.; Walker, J. A.; Vickerman, K. L.; Stanley, L. M.; Jarboe, D.; Wang, T. “Synthesis and characterization of soybean oil-based waxes and their application as paraffin substitute for corrugated coating” Ind. Eng. Chem. Res. 2018, 56, 16371–16375. DOI: 10.1016/j.jiec.2017.09.015
41. Li, X.; Zhang, B.; Tang, L.; Tian, W. G.; Qi, S.; Volkov, A.; Pei, Y.; Qi, Z.; Tsung, C.; Stanley, L. M.; Huang, W. “Cooperative Multifunctional Catalysts for Nitrone Synthesis: Platinum Nanoclusters in Amine‐Functionalized Metal–Organic Frameworks” Angew. Chem. Int. Ed. 2017, 58, 113–122. DOI: 10.1002/anie.201710164
40. Vickerman, K. L.; Stanley, L. M. “Catalytic, Enantioselective Synthesis of Polycyclic Nitrogen, Oxygen, and Sulfur Heterocycles via Rh-Catalyzed Alkene Hydroacylation” Org. Lett. 2017, 19, 5054–5057. DOI: 10.1021/acs.orglett.7b02230

39. Kadam, A. A.; Ellern, A.; Stanley, L. M. “Enantioselective, Palladium-Catalyzed Conjugate Additions of Arylboronic Acids to Form Bis-benzylic Quaternary Stereocenters” Org. Lett. 2017, 15, 4062–4065. DOI: 10.1021/acs.orglett.7b01825
38. Walker, J. A.; Vickerman, K. L.; Humke, J. N.; Stanley, L. M. “Ni-Catalyzed Alkene Carboacylation via Amide C–N Bond Activation” J. Am. Chem. Soc. 2017, 30, 10228–10231. DOI: 10.1021/jacs.7b06191
37. Metz, T. L.; Evans, J.; Stanley, L. M. “Catalytic Conjugate Addition of Electron-Rich Heteroarenes to ß,ß-Disubstituted Enones” Org Lett. 2017, 13, 3442-3445. DOI: 10.1021/acs.orglett.7b01402
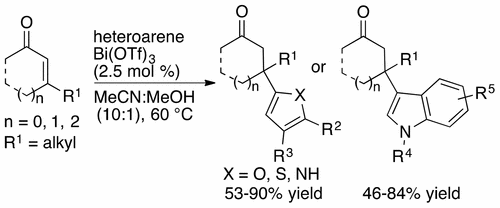
36. Gao, M.; Cao, M.; Suastegui, M.; Walker, J. A.; Rodriguez-Quiroz, N.; Wu, Y.; Tribby, D.; Okerlund, A.; Stanley, L. M.; Shanks, J. V.; Shao, Z. “Innovating a Nonconventional Yeast Platform for Producing Shikimate as the Building Block of High-Value Aromatics” ACS Synth. Biol. 2017, 6, 29-38. DOI: 10.1021/acssynbio.6b00132
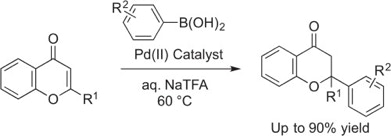
35. Gerten, A. L.; Stanley, L. M. “Palladium-catalyzed conjugate addition of arylboronic acids to 2-substituted chromones in aqueous media” Tetrahedron Lett. 2016, 49, 5460-5463. DOI: 10.1016/j.tetlet.2016.10.084
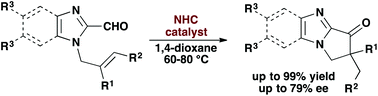
34. Walker, J. A.; Stanley, L. M. “N-Heterocyclic carbene-catalysed intramolecular hydroacylation to form basic nitrogen-containing heterocycles” Org. Biomol. Chem. 2016, 42, 9981-9984. DOI: 10.1039/C6OB01956K
33. Johnson, K. F.; Schneider, E. A.; Schumacher, B. P.; Ellern, A.; Scanlon, J. D.; Stanley, L. M. “Rhodium-Catalyzed, Enantioselective Intramolecular Hydroacylation of Trisubstituted Alkenes” Chem. Eur. J. 2016, 44, 15619–15623. DOI: 10.1002/chem.201603880
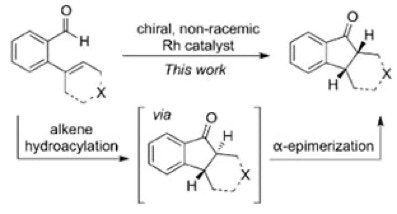
32. Li, X.; Van Zeeland, R.; Maligal-Ganesh, R. V.; Pei, Y.; Power, G.; Stanley, L. M.; Huang, W. “Impact of Linker Engineering on the Catalytic Activity of Metal-Organic Frameworks Containing Pd(II)-Bipydridine Complexes” ACS Catal. 2016, 6, 6324-6328. DOI: 10.1021/acscatal.6b01753
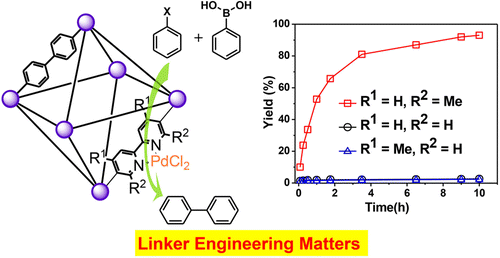
31. Ghosh, A.; Bainbridge, D. T.; Stanley, L. M. “Enantioselective Model Synthesis and Progress toward the Putative Structure of Yuremamine” J. Org. Chem. 2016, 81, 7945-7951. DOI: 10.1021/acs.joc.6b01730
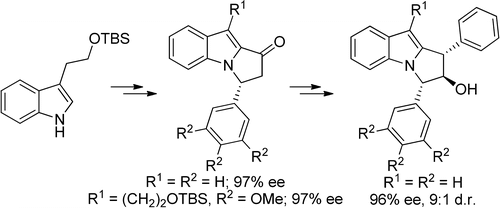
30. Ghosh, A.; Walker Jr., J. A.; Ellern, A.; Stanley, L. M. “Coupling Catalytic Alkene Hydroacylation and α-Arylation: Enantioselective Synthesis of Heterocyclic Ketones with α-Chiral Quaternary Sterocenters” ACS Catal. 2016, 6, 2673-2680. DOI: 10.1021/acscatal.6b00365
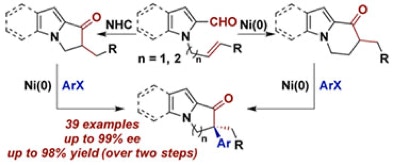
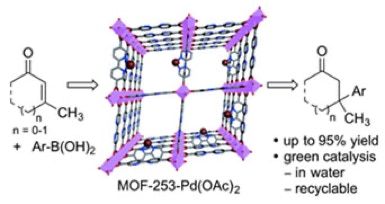
29. Van Zeeland, R.; Li, X.; Huang, W.; Stanley, L. M. “MOF-253-Pd(OAc)2: A Recyclable MOF for Transition-Metal Catalysis in Water” RSC Adv. 2016, 6, 56330-56334. DOI: 10.1039/C6RA12746K
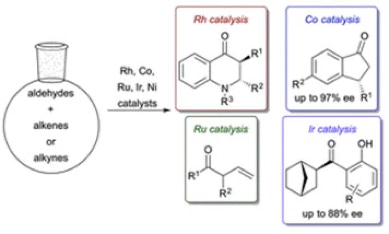
28. Ghosh, A.; Johnson, K. F.; Vickerman, K. L.; Walker, J. A.; Stanley, L. M. “Recent Advances in Transition Metal-Catalysed Hyroacylation of Alkenes and Alkynes” Org. Chem. Front. 2016, 3, 639-644. DOI: 10.1039/C6QO00023A
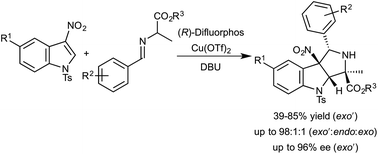
27. Gerten, A. L.; Stanley, L. M.; “Enantioselective Dearomative [3+2] Cycloadditions of Indoles with Azomethine Ylides Derived from Alanine Imino Esters” Org. Chem. Front. 2016, 3, 339-343. DOI: 10.1039/C5QO00346F
26. Johnson, Kirsten F.; Schmidt, Adam C.; Stanley, L. M.; “Rhodium-Catalyzed, Enantioselective Hydroacylation of ortho-Allylbenzaldehydes” Org. Lett. 2015, 50, 2765-2768. DOI: 10.1021/acs.orglett.5b02559

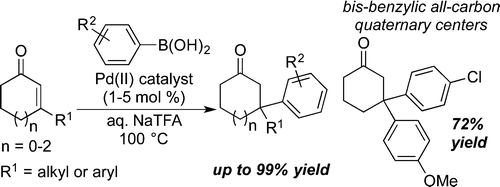
25. Van Zeeland, Ryan; Stanley, Levi M. “Palladium-Catalyzed Conjugate Addition of Arylboronic Acids to ß,ß-Disubstituted Enones in Aqueous Media: Formation of Bis-benzylic and ortho-Substituted Quaternary Centers” ACS Catal. 2015, 5, 5203-5206. DOI: 10.1021/acscatal.5b01272
24. Du, X.W.; Stanley, L. M. “Tandem Alkyne Hydroacylation and Oxo-Michael Addition: Diastereoselective Synthesis of 2,3-Disubstituted Chroman-4-ones and Fluorinated Derviatives” Org. Lett. 2015, 17, 3276-3279. DOI: 10.1021/acs.orglett.5b01447
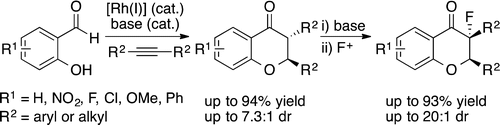
23. Du, X.W.; Ghosh, A.; Stanley, L. M. “Enantioselective Synthesis of Polycyclic NitrogenHeterocycles by Rh-Catalyzed Alkene Hydroacylation: Constructing Six-Membered Rings in the Absence of Chelation Assistance” Org. Lett. 2014, 16, 4036-4039. DOI: 10.1021/ol501869s
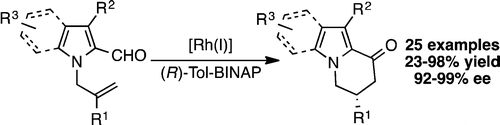
22. Ghosh, A.; Stanley, L. M.; “Enantioselective hydroacylation of N-vinylindole-2-carboxaldehydes” Chem. Commun. 2014, 50, 2765-2768. DOI: 10.1039/c4cc00210e
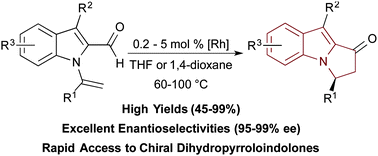
21. Gerten, A.L.; Slade, M.C.; Pugh, K.M.; Stanley, L. M.; “Catalytic, enantioselective 1,3-dipolar cycloadditions of nitrile imines with methyleneindolinones” Org. Biomol. Chem. 2013, 11, 7834-7837. DOI: 10.1039/c3ob41815d
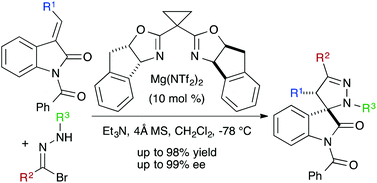
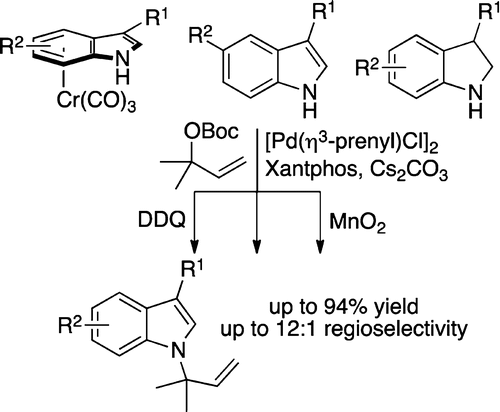
20. Johnson, K.F.; Van Zeeland, R.; Stanley, L. M.; “Palladium-Catalyzed Synthesis of N-tert-Prenylindoles” Org. Lett. 2013, 15, 2798-2801. DOI: 10.1021/ol4011344
Designed by Katerina Sosa