The depletion of fossil fuels and the serious environmental problems resulting from their combustion are two critical challenges facing society. The understanding and development of high performance catalytic systems can effectively address these problems in two ways: (i) by reducing energy consumption through efficient conversion of reactants to desired products, and (ii) by increasing the effectiveness of the methods by which energy is obtained from renewable energy sources. The ultimate goal of the Huang group is to understand and develop integrated catalytic systems based on controlled nanostructures to improve the efficiency of catalytic processes and renewable energy-related reactions.
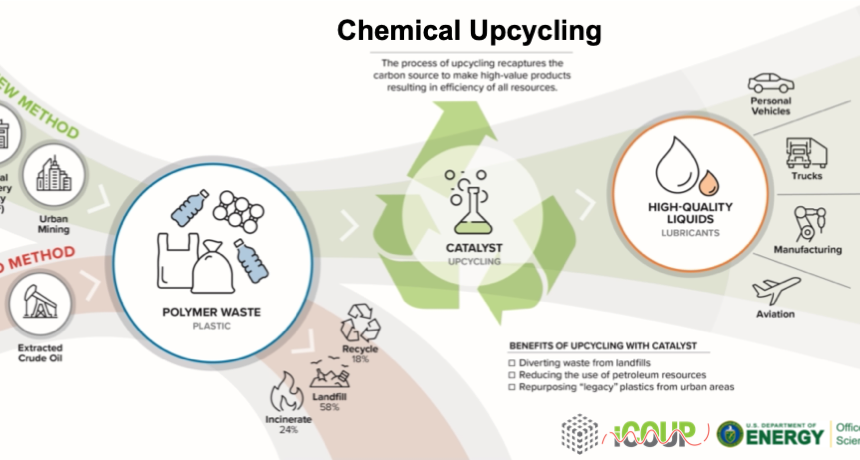
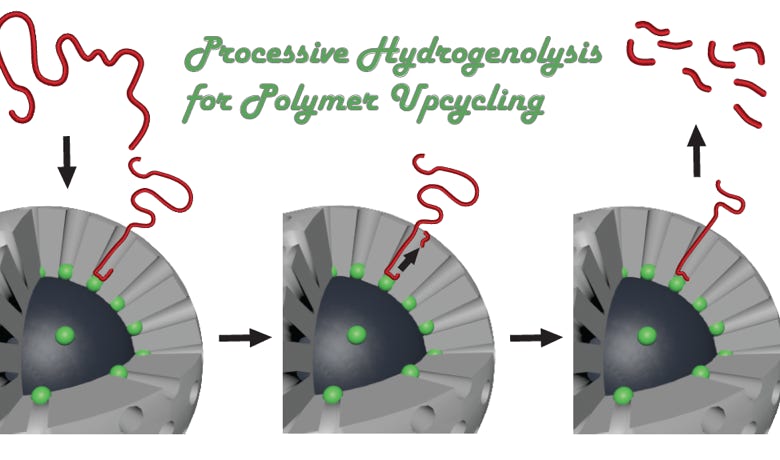
Chemical Upcycling of Waste Plastics using Well-Defined Porous Catalysts
1
Polymers are irreplaceable in the global economy, with myriad uses in packaging, construction, transportation, electronics, and healthcare industries. However, their massive-scale manufacture, single-use function, long lifetimes, slow decomposition rates, and disruption of sensitive ecosystems have created a crisis of plastic waste. Unfortunately, conventional mechanical recycling methods are limited by considerable technological and economic challenges. Chemical upcycling, an emerging alternative to the classical recycling approach, would use plastic waste as a feedstock to synthesize value-added chemicals and materials. This project focuses on developing advanced catalysts that can achieve efficient and selective upcycling of waste plastics to high-value chemicals. Learn more here.
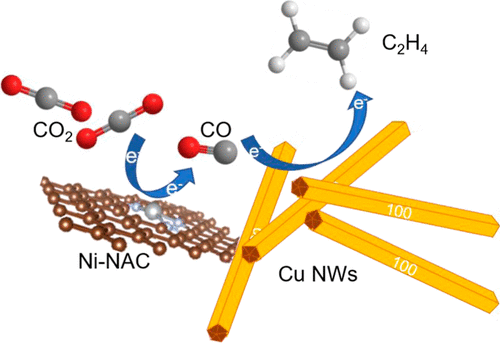
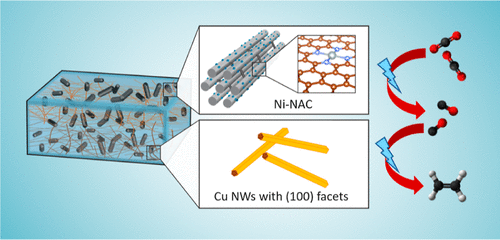
Electrocatalytic Carbon Dioxide Reduction
2
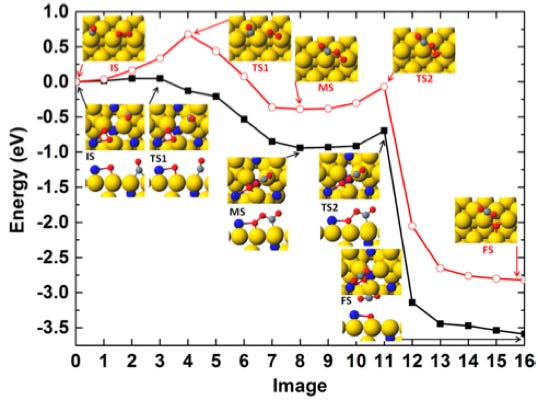
The electrochemical carbon dioxide reduction reaction (CO2RR) is crucial for reducing the carbon footprint, which aligns with the emerging tech revolution towards carbon neutrality and a cyclic economy. Catalytic CO2 transformation reduces footprints, reshaping chemical manufacturing with sustainable chemical production. Electrocatalytic CO2RR, an emerging technology, has captured significant attention for its transformative capabilities. Scaled application entails energy-efficient, readily scalable catalytic systems that utilize earth-abundant elements. Our research delves into engineering material properties, establishing selective CO2 reduction towards desired products. This drives us toward a sustainable, eco-friendly future, emphasizing electrochemical carbon dioxide reduction.
Well-defined Heterogeneous Catalysts based on MOF-confined Nanoclusters
3
Metal-organic frameworks (MOFs) are unique support materials for metal nanoclusters (NCs) that have great potential due to their structural diversity, flexibility, tailorability, and high porosity. A wide spectrum of applications for MOF-based materials have been demonstrated in gas storage, chemical adsorption and separations, catalysis, sensors, and drug delivery. Metal NCs and nanoparticles supported on MOFs have been used as heterogeneous catalysts in many reactions, such as hydrogenation, oxidation, and carbon-carbon bond coupling. However, the diameters of most of these NCs and nanoparticles are larger than the cavities of the MOFs, which indicate that the nanoparticles are mostly supported on the external surface of the MOFs. To fully utilize the benefit of the geometrically and chemically uniform cavities of MOFs, we need to fully confine the catalytic NCs inside the cavities of MOFs. With NCs confined inside the cavities of MOFs, we could fully realize the benefit to have MOFs as supports for metal NC catalysts.
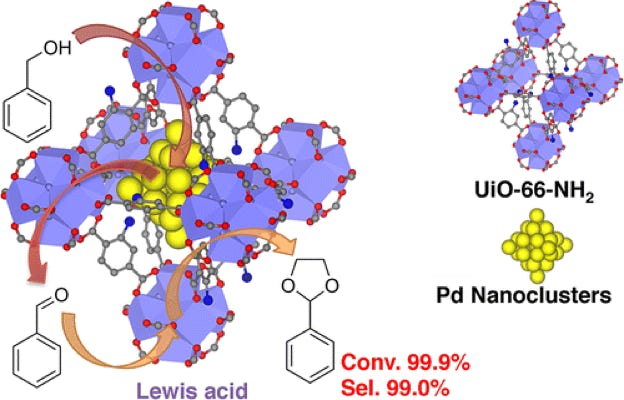
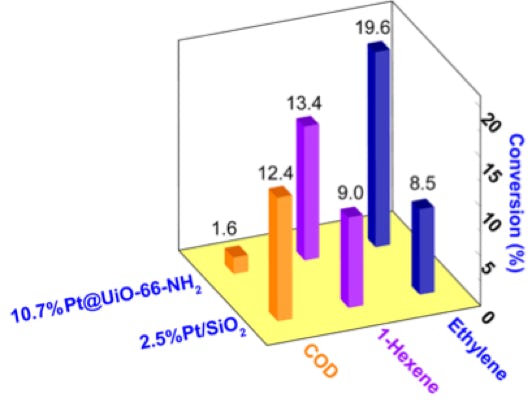
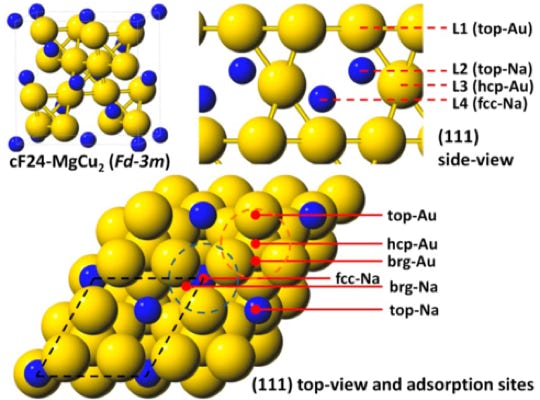
Well-defined Heterogeneous Catalysts based on Intermetallic Compounds
4
Precious metals and metal alloys are important heterogeneous catalysts for renewable energy and materials. However, both of them have their limitations. Precious metals have low natural abundance and are expensive. Metal alloys have unstable surfaces due to surface segregation under reaction conditions, which renders the identification of active sites and the understanding of reaction mechanisms difficult. My research group will address these limitations by developing new intermetallic NP catalysts. Intermetallic compounds, which consist of two or more metallic elements, adopt specific crystal structures as well as electronic structures different from the constituent elements. The modified electronic structures of intermetallic compounds make them unique catalytic materials. It has been proposed that such compounds should be treated as new “elements” considering their potential in catalysis. The inherent properties of intermetallic compounds, stable and exhibit a large variety of structures, will help us to discover catalysts with stable surfaces, consisting of more abundant metals, to replace unstable alloy and precious metal catalysts.