pH Meter - Instrument

What does measuring pH help us know?
It's easy to take for granted that pH meters exist and are fairly easy to use. If we think in more historical terms, however, it's actually pretty amazing. Click here for a page that talks about how the pH meter lets us investigate nature in ways that early chemists wouldn't even dream of.
How does a pH meter work?
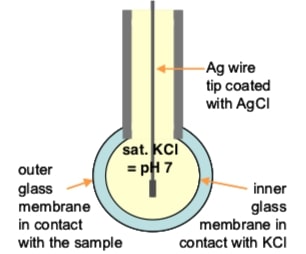
A pH meter is consisted of three different parts: an internal electrode, a reference electrode, and a high input impedance meter. Glass probe often contains the two electrodes -- internal electrode and reference electrode. The internal electrode is a Silver wire covered with Silver Chloride (Ag/AgCl wire), and reference electrode is often made up of the same materials. Inside the probe is a buffer solution at pH of 7. Measured pH is the difference in [H+] between the reference buffer inside the probe and the sample solution.
The pH measurements are made by comparing the pH reading of a sample solution to that of a reference solution with defined pH, such as buffers. Therefore, it is important to calibrate the instrument with appropriate buffer solutions before making any measurements. The figure on the right is a simple depiction of a glass electrode used with pH meters (Source: Electrochemistry Theory and Practice, Hach).
For more information on calibration, visit the calibration page.
What does a pH meter measure?
An electronic pH meter is used to obtain more accurate pH measurements. A pH meter is an instrument used to measure hydrogen ion activity in solutions - in other words, this instrument measures acidity/alkalinity of a solution. The degree of hydrogen ion activity is ultimately expressed as pH level, which generally ranges from 1 to 14.
This pH measurement is directly related to the ratio of hydrogen ion concentration and hydroxyl ion concentration ([H+] and [OH-], respectively). The general breakdown of pH levels is listed below:
- Neutral solution: pH = 7
- Acidic solution: pH < 7
- Basic solution: pH > 7
A neutral solution will show a pH of 7 due to the equal activities of hydrogen and hydroxide ions. Acidic solutions exhibit pH readings below 7 (higher hydrogen ion activity compared to hydroxide ion activity), and basic (or alkaline) solutions exhibit pH levels above 7 (hydroxide ion activity is greater than that of hydrogen ion).
Applications
pH meters are widely used in the following industries: 
- Food & beverage
- Pharmaceutical
- Oil & gas
- Agriculture
- Water treatment plant
While this is not an exhaustive list of industries utilizinig pH meters, it is clear that pH meter is an essential component of science and that it has a big impact on our lives. For example, they can be used to measure acidity levels in wastewater, which is a vital component of wastewater treatment process. They are used to analyze the exact pH value of food grade products and chemical products to ensure safety and quality, or they can be used to evaluate acidity/alkalinity of drugs in pharmaceutical and biotechnology industries.