Conductivity Meter

What does conductivity meter measure?
Conductivity meter allows us to measure the level of conductivity in solutions. Conductivity is an ability of materials (solutions, metals or gases) to pass an electric current. While all materials possess the ability to pass electric currents, the degree of such ability can vary. Substances with conductive aqueous solutions are referred to as electrolytes. These electrolytes are able to break down into ions when dissolved in water, thus creating free ions in the solution. Acids, bases, and salts are examples of electrolytes. Substances with non-conductive aqueous solutions are referred to as nonelectrolytes. These substances are often composed of covalent bonds, and examples include Carbon-containing compounds, fat, and sugar.
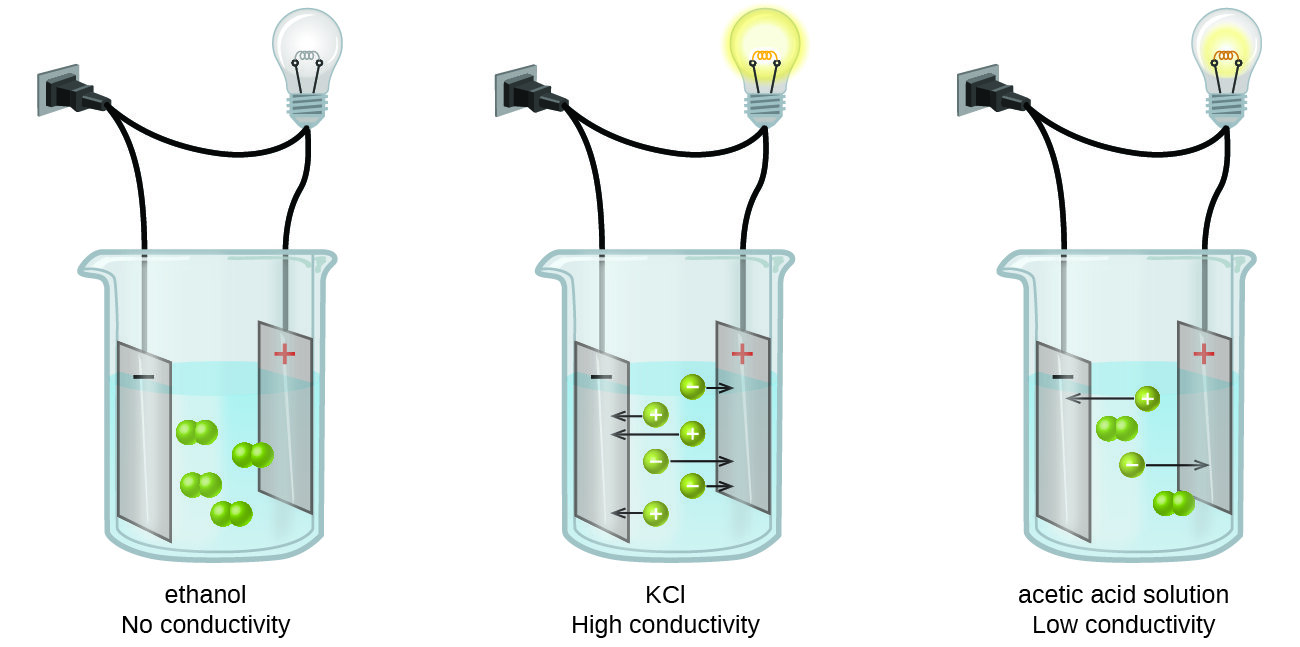
Solutions with high concentration of ions will exhibit high conductivity. On the other hand, solutions with low concentration of ions will result in small conductivity reading. A few factors that effect conductivity measurement are temperature, concentration of ions, and the nature of ions present in the solution.
The figure shows the meaning of conductivity with three different examples. Nonelectrolyte substance, ethanol, does not produce free ions in solution and therefore cannot conduct electricity. Potassium Chloride (KCl) is a strong electrolyte that completely ionizes in water, thus possessing high conductivity. Acetic acid is a weak electrolyte that does not fully ionize in water, conducting low amount of electricity. (Figure source: https://opentextbc.ca/chemistry/chapter/11-2-electrolytes/)
Applicatons
Conductivity measurements are important in many aspects of life, not just in scientific inquiries. Conductivity meters are used widely in areas such as water treatment and agricultural industries. Here are some examples of conductivity meter application found in real life:
- Used to evaluate the quality of water - is the water drinkable or usable for irrigation/industry?
- Measure the quality of water in wastewater facility for treatment - corrosion measurement, etc
- Water pollution monitoring
- Measure salinity of the surface water - important for wildlife, aquatic plants, and agriculture
- Evaluate purity of water used for pharmaceuticals, cosmetics, food, etc
For more information on how knowing conductivity of aqueous solutions help us understand the world around us in more detail, click here.
How does a conductivity meter work?
A conductance of a solution can be measured by applying an alternating electrical current to the two electrodes present in the probe. While this electrical current is applied to the solution, the cations (ions with a + charge) move to the negative electrode and the anions (ions with a - charge) move to the positive electrode. This movement of the ions leads to the solution to be conductive.
For more information on calibration, visit calibration page.
drawFooter() ?>